News Updates
NEWS CENTER
Related News
There is currently no data available
China's first oral anti-COVID-19 drug with independent intellectual property rights has been approved for sale
Release Time:
2022-07-28
According to the website of the State Food and Drug Administration, on July 25, according to the relevant provisions of the Drug Administration Law, the State Food and Drug Administration conducted an emergency review and approval in accordance with the special drug approval procedure, and conditionally approved the application for azvudine tablets of Henan Real Biotechnology Co., Ltd. to increase the indication registration for the treatment of new coronavirus pneumonia. Azvudine tablet is an oral small-molecule novel coronavirus pneumonia treatment drug independently developed in China. On July 20, 2021, the State Drug Administration has conditionally approved the use of this product in combination with other reverse transcriptase inhibitors for the treatment of adult HIV-1 infected patients with high viral load. This is a conditional approval of the new indication for the treatment of adult patients with common novel coronavirus pneumonia (COVID-19). Patients should strictly follow the instructions under the guidance of doctors.
According to the website of the State Food and Drug Administration, on July 25, the State Food and Drug Administration conducted emergency review and approval in accordance with the relevant provisions of the Drug Administration Law, in accordance with the special drug approval procedure.Conditionally approved the application for the registration of Azvudine tablets for the treatment of novel coronavirus pneumonia by Henan Real Biotechnology Co., LTD.
Azvudine tablet is an oral small-molecule novel coronavirus pneumonia treatment drug independently developed in China.On July 20, 2021, the State Drug Administration has conditionally approved the use of this product in combination with other reverse transcriptase inhibitors for the treatment of adult HIV-1 infected patients with high viral load.This is a conditional approval of the new indication for the treatment of adult patients with common novel coronavirus pneumonia (COVID-19).Patients should strictly follow the instructions under the guidance of doctors.
The National Food and Drug Administration requires the marketing authorization holder to continue the relevant research work,deadline for completion of conditional requirements,Submit follow-up research results in a timely manner.
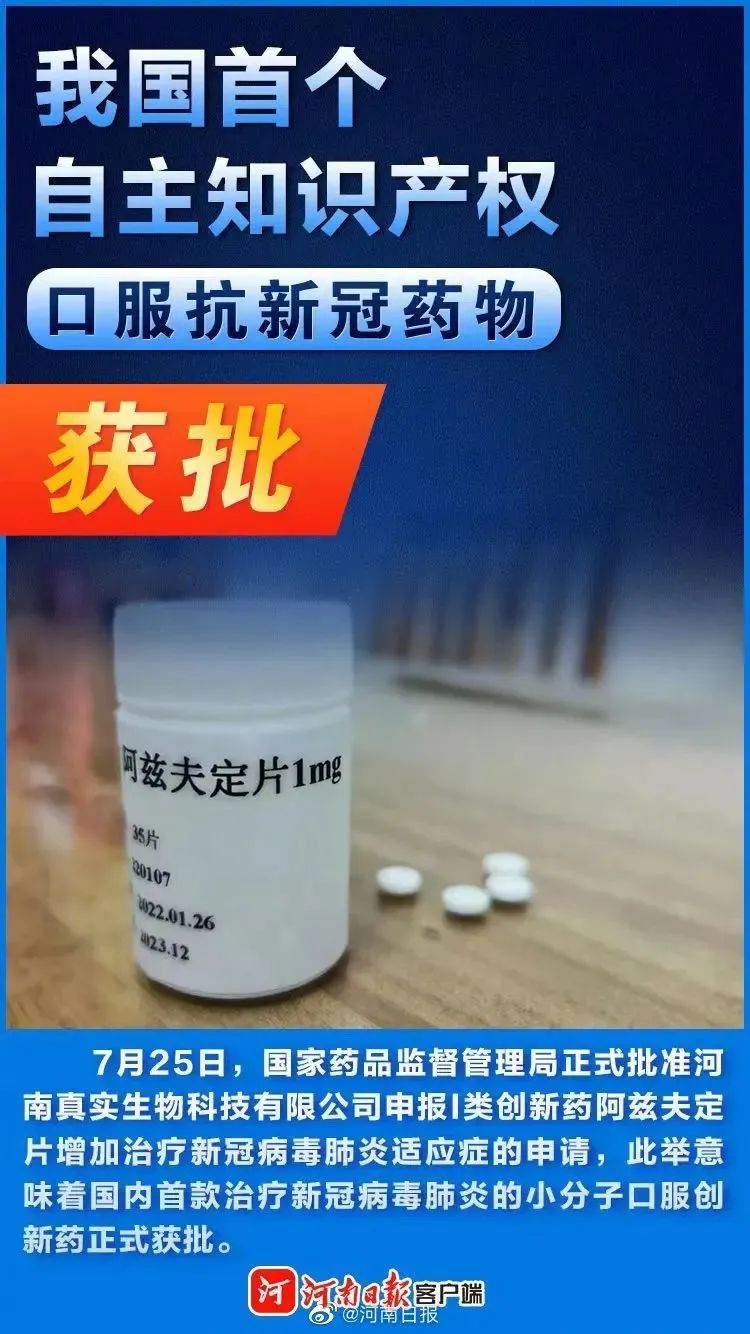
Henan Daily reported that the State Medical Products Administration officially approved Henan True Biotechnology Co., LTD. 's application to apply for a new type I innovative drug Azvudine tablet for the treatment of novel coronavirus pneumonia,This means that China's first small molecule oral innovative drug to treat the novel coronavirus pneumonia has been officially approved.
At present, Azfudine from research and development, API production, preparation production, to the sales headquarters and other whole industry chain has the basic conditions for landing in Henan.
Conditional approval
According to the Drug Administration Law of the People's Republic of China, for the treatment of seriously life-threatening diseases without effective treatment means and for drugs urgently needed in public health, clinical trial data of drugs showing efficacy and predicting their clinical value may be approved with conditions, and the relevant matters shall be stated in the drug registration certificate.
For conditionally approved drugs, the holder of the drug marketing authorization shall take corresponding risk management measures, and complete the relevant research in accordance with the requirements within the prescribed time limit; If the research fails to be completed in accordance with the requirements within the time limit or the benefits cannot be proved to outweigh the risks, the drug regulatory department under The State Council shall handle the matter according to law until the drug registration certificate is cancelled.
Source: Beijing Daily, State Food and Drug Administration
More intellectual property information and services
Pay attention to [Shenkexin Intellectual Property] Official subscription number

More deeply credible dynamic news, important data/report/case, etc.
Pay attention to the [Shenkexin Intellectual Property Service Platform] official service number

相关新闻
Telephone:
Telephone:+86-755-82566227、82566717、13751089600
Head Office:13 / F, Building 14, Longhua Science and Technology Innovation Center (Mission Hills), No. 8 Golf Avenue, Guanlan Street, Longhua District, Shenzhen
Head Office:
13 / F, Building 14, Longhua Science and Technology Innovation Center (Mission Hills), No. 8 Golf Avenue, Guanlan Street, Longhua District, Shenzhen
Subsidiary Company:2808, Block B2, Yuexiu Xinghui Junbo, No.18 Tazihu East Road, Jiangan District, Wuhan City, Hubei Province
Subsidiary Company:
2808, Block B2, Yuexiu Xinghui Junbo, No.18 Tazihu East Road, Jiangan District, Wuhan City, Hubei Province

Service Number

Subscription Number
Copyright ©2016 Shenzhen Shenkexin patent Agency Co., LTD All rights reserved | 粤ICP备2021174526号
Copyright ©2016 深圳市深可信专利代理有限公司 版权所有 | 粤ICP备2021174526号 SEO标签
Copyright ©2016 深圳市深可信专利代理有限公司 版权所有